Technical Information
8. Information about temperature measurements with PtRh thermoelectric-elements
Contents
Influences of the environment, thus from protection ceramics, can change the thermoelectric voltage even of precious metal thermocouples. Above all in oxidising and reducing atmospheres from 1300°C iron impurities of the ceramics lead to incorrect measurements. In reducing atmosphere as little as 0,2% Si causes swift embrittlement and changes of the thermoelectric voltage. The use of Aluminium- Oxide- thermowells with 99,7% Al2O3 is therefore necessary.
In research and production, temperatures are preferably measured with thermocouples. Above 1200°C the precious metals are unrivalled because of their oxidation- and corrosion stability. The elements from a platinum basis (Pt-Pt 10% Rh von le Chatelier1, Pt 6% Rh-Pt 30% Rh) have attained the greatest importance. It is however often overlooked in practice, that PtRh- thermocouples don’t guarantee dependable measurements especially over a long period and without careful control, considerable measurement errors or indeed premature failure can occur.
As reasons for a change of the thermoelectric voltage in application, essentially three influences come into question:
- Change of the composition of the leg by diffusion over the soldered connection.
- Change of the composition of one or both legs through selective evaporation of a core component.
- Change of the composition of one or both legs through taking up foreign elements from the environment. Here, besides the oven atmosphere, the protection ceramics play a considerable role
1. Modification of the thermoelectric voltage through inter- diffusion
The thermovoltage of a material against a reference element depends sensitively on its composition. The use of precious metal thermocouples generally lie in a temperature range, in which solid reactions and diffusion processes can already proceed to a considerable extent, so that a constant composition of the thermocouple is no longer ensured. A possible cause for a change of the composition is the inter-diffusion of the elements of the two legs. Since generally, through a ceramic capillary staff a core formation over the gaseous phase, is to a large extent prevented, the core formation is limited to the soldered connection.
2. Modification of the thermovoltage through selective evaporation
Different binding energies and thus different evaporation rates of the two core elements of a leg change the concentration of the thermocouple wire. In2 a series of works on intensified Rhodium evaporation from Pt-Rh- thermocouples is reported . This contradicts the work of McQuillan3, who likewise finds substantial decreases in weight of the thermocouple wires, but identifies the evaporated component however as platinum. At a Pt-13% Rh element the author thereby measures after an annealing at 1600°C at air a decrease in weight of 10,3%. The evaporated quantity of platinum results in a change of concentration of the wire, so that we are now dealing with a Pt-14.5% Rh- element. This change corresponds according to figure 1. 1 from4 a change of the thermoelectric voltage of about 1mV, i.e. a faulty measurement of almost 100°C for a measurement against platinum. This simple appraisal shows the importance of these effects. According to3 for annealings in the vacuum the evaporation rates lay in the same order of magnitude, in contrast, in reducing atmospheres, the decrease in weight was substantially smaller.
3.Changes of the thermovoltage through environmental influences.
The most serious influence is, in practice, the respective environment of the thermocouples: Entering foreign matter changes the thermoelectric strength of the elements or causes by formation of second phases a premature break of the thermoelectric element. Particularly dangerous here in reducing atmospheres are arsenic, phosphorus, sulphur, silicon, and boron, which through the formation of eutectic phases even leads to heat brittleness when red hot. For this reason one protects thermocouples by closed ceramic insulating tubes. The influences of the oven chamber can therefore be disregarded and the following executions be limited to the effect of the ceramic thermowells on the elements. Prerequisite for the disregarding of other influences however is highest cleanliness during assembly , because oils, fats (sulphur!) or metallic impurities can lead to rapid subsequent damages.
3.1 Influences in oxidised atmosphere
The influence of ceramic masses on the thermoelectric characteristics was examined for the first time by Chaussain6. He bedded Pt-wires into ceramic powders and measured the change of the thermovoltage with the glow time. He found SiO2 as the most harmful material, followed by CaO, Al2O3, ZrO2, MgO and ThO2 as best material. Ehringer4s defined the thermoelectric change for the materials of the PtRh10% Rh and of the PtRh 18 element, with the insertion time in different ceramic powders. As substances he used pure Alumina (99.5% Al2O3, remainder of SiO2, Fe203, MgO, Na20), a mullitic material and silicon dioxide. Ill. 2 shows it’s results for an annealing at air at 1400°C. It results that even after 50h employment in Alumina no substantial changes arise, while in mullitic materials and still more in SiO2 deviations are found. These changes correspond to a faulty measurement of 10°C and/or 4°C for PtPt 10% Rh and PtRh 18 in the aluminium silicate and, in the SiO2 a faulty measurement of 30°C and/or 20°C. This influence of the SiO2 was examined more closely by Prospisil. He found thereby that the acceptance of the EMF at air is to due not on an effect of the SiO2, but is traceable to iron impurities in the silicon dioxide. In table 1 it’s results for the relative change of the thermoelectric voltage of platinum are represented after 24h reaction at 1300°C with different materials. The mullitic materials, which all contain SiO2 and iron, cause an increase of the thermoelectric voltage of platinum, i.e. a reduction of the thermoelectric voltage of the element. One sees further the intense change with technical quartz and the harmlessness of highly pure quartz.
| Ceramic | Change E in % |
|---|---|
| Quartz | 0,00 |
| Al2O3 | -0,05 |
| Corundum (95% Al2O3) | -0,06 |
| MgO | -0,06 |
| Pythagoras (Multi material) | +0,25 |
| Techn. quartz (Highly purified) | +0,35 |
| Triangle (Multi material) | +0,37 |
| Signodur (Multi material) | +0,60 |
| Multi CZ | +0,61 |
| Technical quartz | +0,70 |
| 1,0% Na2O in Al2O3 | -1,76 |
| 2,5% FeO in Al2O3 | +2,96 |
| 2,5% Fe2O3 in Al2O3 | +5,52 |
Tab. 1: Changes of the thermocouple voltage E from platinum after 24h contact with different ceramics at air at 1300°C (from Proposil 7)
The mixture of the ferric oxides with Al203 are an indication that the iron causes the observed effect. It is also interesting that the author after 8000h at about 1300°C in a mullitic thermowell found an incorrect measurement of 40°C (all the pre named numbers refer to measurements, by which the thermoelements through the powder packing came more intensively in contact with the ceramics). The author hereby points out that a relative change of the thermoelement in the oven can result in considerably incorrect measurements.
3.2 Influences in a neutral atmosphere
We get substantial findings about the influences of protection ceramics in a neutral atmosphere from a work of Walker8. The authors measured the change of the thermoelectric voltages of PtRh alloys in contact with different qualities of Alumina. Other influences were excluded by a series of parallel attempts. Two independent analyses of the analysed protection ceramics on iron and silica produce the result that the parts of both elements in the materials always change about evenly. From these analyses no conclusion can be drawn about the responsible impurities. Spectro chemical investigations of the wires after annealing yielded information . The ferric content of the Pt and PtRh wires had increased considerably and, for platinum, was approximately proportional to the measured change of the EMF. In contrast a recording of sodium or silicon could not be recorded . By further annealings in powder mixtures with Al203, SiO2 and Fe203 these authors also conclude that iron impurities are responsible for the observed changes of the thermovoltage. The bad performance of platinum compared with it’s cores is based on a higher sensitivity in relation to impurities, since the ferric content was about alike for all wires after annealings. Some investigations of the authors with the same systems at air resulted in the fact that the observed changes were substantially smaller than in Argon, but the effects were alike in quality however.
3.3 Influences in reducing atmosphere
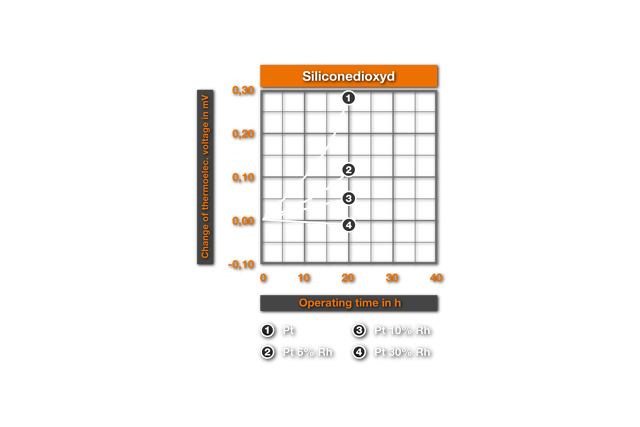
The thermoelectric changes described above occur very swiftly and more intensely in reducing environment. Ill. 4. from4 shows the Ill.2 analogue results for a 1400°C-annealing under hydrogen (thermoelectric changes measured at 1200°C). One recognizes that here mullitic materials and silicon oxide can no longer be used for protection ceramics, as within minutes (changed Apsis scale!) strong thermoelectric changes and embrittlements arise. Also on use of the "pure" Alumina particularly in non cored platinum, a rapid change of the thermoelectric strength occurs, which leads to the faulty measurement. The PtRh 18-Element shows up to be clearly over core here. The cause of this behaviour is that the SiO2 of the protection ceramics, that is reduced by hydrogen to the gaseous SiO, which reacts with platinum to the Silicide Pt5Si2 (melting point 830°C). Grain boundary extractions of this Silicide lead to the observed changes. Experimentally this explanation was confirmed by Bennet9, which proved the occurrence of the grain boundary phase metallographically. Particularly remarkable thereby was his result, that even SiO2-impurities in the order of magnitude of 0,2% are even sufficient for the formation of embrittling Silicide in so-called pure Alumina . This explains the changes represented in Ill. 4 with annealing in pure Alumina, since here according to the data it deals with a ceramic with 99,5% Alumina. For the protection of PtRh- thermocouples in reducing atmospheres only purest Al203-ceramics with 99,7% Al203 (remainder of MgO, SiO2, Na2O come into question .Thus Bennett did not find damage even after one year at 1400°C.
Literature
- Le Chatelier genie Civil X, 18, März 1887
- Temperature, Ist Measurement and Conrol in Science and Industry, Reinhold Publishing Corporation New York 1941
- M.K. McQuillan I.Sci. Instr.26 (1949) 329-331
- H.Ehringer Metall 8 (1954) (15/16) 596-598
- Ullmanns Bd.14. S.33, Encyclopädie der Technischen Chemie 3. Auflage
- M.Chaussain Fonderie 77 (1952) 2955
- Z.Pospisil Silikat Journal 7 (1968) 140-142
- B.E.Walker et al Rev.Sci.Instr. 33 1962 (10) 1029-1040
- H.E.Bennett Platinum Metals Rev.5. 1961 (4) 132-133
Source: W. Haldenwanger "Masses, Rules, Technical Ceramics"
Popular Pages
Products Resistance Thermometers (-200 °C to 850 °C) 6X-BTF - 6X-BTF. Temperature probes with bayonet lock 7X-KFW - Cable resistance thermometers Thermocouples (up to 2300 °C) MarineTECH GML-R - GML-R Resistance thermometers series MarineTECH MarineTECH GML-T - GML-T Thermocouples serii MarineTECH Calibratable sensors GT 5333BL Indicators, recorders, meters Compensation and thermoelectric cables Service Temperature uniformity survey Laboratory Our company News DownloadsYOUR DIRECT CONTACT TO OUR TEAM
Guenther Polska Sp. z o.o.
Ul. Wrocławska 27C
55-095 Długołęka
Polska
Tel.: 00 48 71 352 70 70
Fax: 00 48 71 352 70 71
E-mail: biuro@guenther.com.pl